We do exactly two things in AP chemistry. Stoichiometry and Coulombic attractions. This unit is the mathematical foundation of almost everything else we will do this year. Your determined mindset to learn, understand and apply the fundamentals of stoichiometry will be rewarded in nearly every unit of learning. Two pencils and maximum effort.
Mole Unit & Conversions
Avogadro’s number provides the connection between the number of moles in a pure sample of a substance and the number of constituent particles (or units) of that substance.
Thus, for any sample of a pure substance, there is a specific numerical relationship between the molar mass of the substance, the mass of the sample, and the number of particles (or units) present.
The number of atoms, molecules, or formula units in a given mass of substance can be calculated.
Molecular Composition
Mass Percent
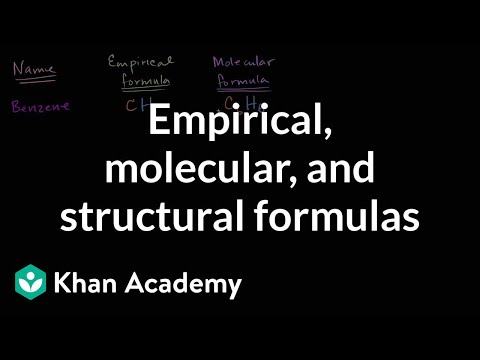
An empirical formula is the lowest whole number ratio of atoms in a compound. Two molecules of the same elements with identical mass percent of their constituent atoms will have identical empirical formulas.
Because pure compounds have a specific mass percent of each element, experimental measurements of mass percents can be used to verify the purity of compounds.
Combustion Analysis
Combustion is the exothermic reaction of a hydrocarbon with oxygen to form carbon dioxide and water.
The empirical and molecular formula of a hydrocarbon is determined from experimental results obtaining the mass of carbon dioxide and water formed from combustion.
The molecular formula of a hydrocarbon is determined given the molecular mass of the hydrocarbon and the results of combustion analysis.
Equation Writing & Types of Reactions
Atoms and molecules interact with one another on the atomic level. Balanced chemical equations give the number of particles that react and the number of particles produced. Because of this, expressing the amount of a substance in terms of the number of particles, or moles of particles, is essential to understanding chemical processes.
The subscripts in a chemical formula represent the number of atoms of each type in a molecule.
The coefficients in a balanced chemical equation represent the relative numbers of particles that are consumed and created when the process occurs.
The concept of conservation of atoms plays an important role in the interpretation and analysis of many chemical processes on the macroscopic scale.
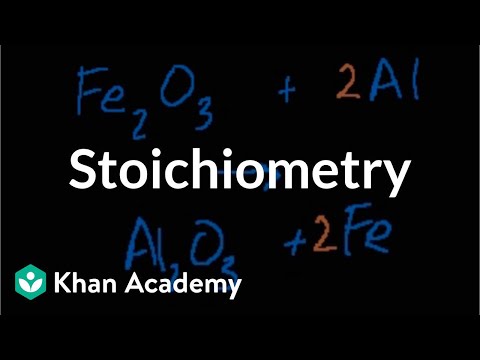
Stoichiometry
The concept of conservation of atoms plays an important role in the interpretation and analysis of many chemical processes on the macroscopic scale.
In gravimetric analysis, a substance is added to a solution that reacts specifically with a dissolved analyte (the chemical species that is the target of the analysis) to form a solid. The mass of solid formed can be used to infer the concentration of the analyte in the initial sample.
Limiting Reactant Stoichiometry
Coefficients of balanced chemical equations contain information regarding the proportionality of the amounts of substances involved in the reaction.
These values can be used in chemical calculations that apply the mole concept; the most important place for this type of quantitative exercise is the laboratory. 1. Calculate amount of product expected to be produced in a laboratory experiment. 2. Identify limiting and excess reactant; calculate percent and theoretical yield for a given laboratory experiment.
Extra Stoichiometry Instructional Videos
Here's another Melissa Maribel playlist for concepts related to stoichiometry. If the Khan Academy videos are too slow or don't work for you, give Melissa's videos a try. Bookmark this playlist! You will most likely want to refer back to it often over the course of the year, especially for midterm review.